Accountants use t accounts in order to make double entry system bookkeeping easier to manage.
Single-step determination of sulfate ions, using desensitized ionene-stabilized gold nanoparticles
Viktoriya V. Arkhipova, Vladimir V. Apyari, Stanislava G. Dmitrienko
Lomonosov Moscow State University, Chemistry department, Leninskie gory 1/3, 119991 Moscow, Russia
ABSTRACT
Desensitized ionene-stabilized gold nanoparticles have been prepared and applied as a colorimetric probe for the single-step determination of sulfate ions at the MPC level. The approach is based on aggregation of the nanoparticles, leading to the change in the absorption spectra and color of the solution; due to both electrostatic and steric stabilization, these nanoparticles are characterized by the decreased sensitivity that allows the simple and rapid direct single-step determination of sulfate at the relatively high concentration level in real water samples without some pretreatment or dilution of a sample. Influence of different factors (the time of interaction, pH, the concentrations of sulfate ions and nanoparticles) on the aggregation and analytical performance of the procedure was investigated. The method allows determination of sulfate ions in the mass range of 0.2 – 0.4 mg with RSD of 5% from the sample volume of less than 2 mL. The time of the analysis is 2 min. The method was applied to the analysis of mineral waters.
Keywords: ionene-stabilized gold nanoparticles, aggregation, sulfate, single-step determination, spectrophotometry, colorimetry
1. Introduction
The determination of sulfate is of general relevance, because it is an important indicator of the water pollution. Human activity such as the combustion of fossil fuels and sour gas leads to significant emissions of sulfur dioxide into the atmosphere. At high levels, sulfuric acid can cause an acidification in the aquatic environment. On the other hand, sulfate is an important component of some mineral waters or pharmaceutical formulations. Thus, objects, in which sulfate needs to be controlled, are quite different. They include sweet, mineral- and sea water, raw materials of chemical industry, food, etc.
Common methods for the determination of SO42-, besides classical gravimetry and turbidimetry, are potentiometry [1 – 3] and ion chromatography [4 – 9]. However, these techniques have certain drawbacks such as the large volume of a sample, high chemicals consumption, bad selectivity in some cases. Ion-chromatographic technique overcomes most of the drawbacks mentioned above. However, this method requires long elution time and high operational cost. Thus, a simple, quick and inexpensive technique for the determination of sulfate at the concentrations near to the permissible levels is still of great importance. The prospective way is spectrophotometric or visual colorimetric analysis.
Recently, gold nanoparticles (NPs) have been suggested as a peculiar chromogenic reagent in spectrophotometry and colorimety. A great number of colorimetric techniques for the determination of different organic compounds [10 – 18], metal ions [19 – 28] and other analytes, based on NPs have been suggested for the past ten years. The AuNPs-based colorimetric platform has also found application for sensing anions including dihydrophosphate [29, 30], hydrophosphate [31], fluoride [32, 33], cyanide [34], nitrite [35], sulfide [36], hypochlorite [37]. AuNPs have been only once previously reported as a colorimetric probe for the detection of sulfate [38]. This assay is sensitive and convenient when detecting trace amounts of sulfate. However, when analyzing real samples (such as natural-, tap-, swimming pool- and mineral waters), the task is often to detect reliably the quite high concentrations of sulfate. In this case, high sensitivity is not needed or even undesirable, as the high-rate dilution of a sample increases the errors and the probability of contamination by exterior substances including sulfate.
Herein, we present a simple single-step colorimetric method for the determination of the relatively high concentrations of sulfate in aqueous solution, using desensitized positively-charged gold nanoparticles stabilized with 6,6-ionene, without any sample preparation.
2. Materials and methods
2.1. Materials
Hydrogen tetrachloroaurate, sodium borohydride, N,N,N,N,-tetramethylhexamethylenediamine, 1,6-dibromohexane, N,N-dimethylformamide, acetone, hydrochloric acid, phosphoric acid, sodium hydroxide, sodium sulfate, potassium sulfate, lithium sulfate, sodium bromide, chloride, perchlorate, chlorate, fluoride, nitrate, phosphate and bicarbonate, ethylenediaminetetraacetate disodium salt (EDTA) were used. All substances were at least of analytical grade. The substances stock solutions were prepared by dissolving their weighted portions in deionized water. 6,6-Ionene (poly(N,N-dimethyl hexamethyleneimine hydrobromide)) was synthesized according to the following procedure [39]:
Equimolar solutions of N,N,N,N,-tetramethylhexamethylenediamine and 1,6-dibromohexane were dissolved in N,N-dimethylformamide up to the final concentration of each substance of 1 mol L-1. The mixture was stirred using a magnetic stirrer at room temperature, until sedimentation of the polymer was completed. The end point of the reaction was controlled visually by the volume of the sediment. The mixture was poured at stirring into 20-fold volume of water-free acetone. The sediment was filtered under suction and washed doubly with 10 mL of acetone. The product was dried under the vacuum of 10-2 mm Hg.
2.2. Instrumentation
Absorption spectra of the solutions were recorded by SF-103 spectrophotometer (Akvilon, Russia), pH was measured by Ekspert 001 ion meter (Ekoniks, Russia), TEM images of the samples and electron diffraction were recorded using LEO912 AB OMEGA microscope (Carl Zeiss, Germany).
2.3. Synthesis of ionene-stabilized gold nanoparticles
Gold nanoparticles stabilized with 6,6-ionene were prepared by reducing metal salt precursor (hydrogen tetrachloroaurate, HAuCl4) in the presence of 6,6-ionene by sodium borohydride.
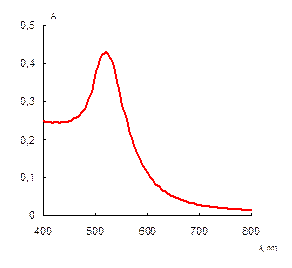
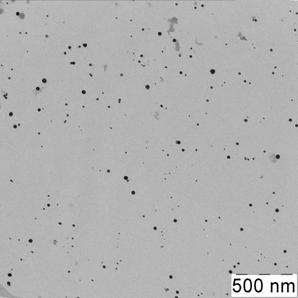
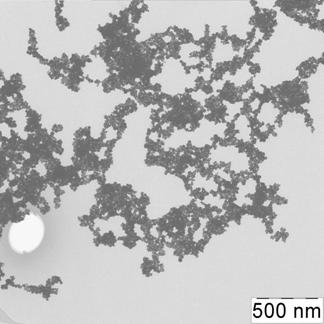
Briefly, 0.05 g portion of 6,6-ionene was placed in a round bottom bulb, dissolved in 18.5 mL of deionized water; 6.5 mL of 0.1 mol·L-1 HCl was injected at stirring. After that 25 mL of a solution containing 1.25 mL of 1 % HAuCl4 was introduced in the bulb dropwise. The brown solution was stirred for 15 min. Then, a solution of 0.025 g NaBH4 in 50 mL of water was added dropwise into the bulb at vigorous stirring. The color of the solution was changed to ruby. The solution was stirred for 30 min and kept for 24 hours to achieve re-crystallization and complete stabilization of NPs. Concentration of NPs in final solution was 70 mg·mL-1 (0.35 mmol·L-1 in terms of gold). Prepared by this technique gold NPs were characterized by UV-vis absorption spectra, transmission electron microscopy (TEM) and electron diffraction (ED) (Fig. 1).
The synthesized ionene-stabilized gold nanoparticles had the average diameter of 16 nm. They were characterized with a surface plasmon resonance (SPR) band in water solution at 520 nm.
2.4. Determination of sulfate
To plot a calibration curve, 0–0.45 mg of sulfate was introduced in a test-tube and diluted with deionized water up to 2 mL. Then, 1 mL of NPs solution (c = 0,35 mmol L-1 Au) was added. The total volume of all components was 3 mL. Absorption spectra were recorded after 2 min of the reaction. The calibration curve was plotted as A650/A520 ratio versus the concentration of sulfate.
3. Results and discussion
The task of the determination of sulfate at the relatively high concentrations, using gold NPs implies solving several problems. The NPs should be positively charged to allow effective interaction with anions, and quite stable to exclude their aggregation at the low concentrations of sulfate and to diminish interferences from single-charged species. We suggest 6,6-ionene as a stabilizer of gold NPs appropriate for this purpose. Being a cationic polymer, 6,6-ionene is adsorbed on gold surface, stabilizing NPs owing to the electrostatic repulsion between positively charged ionene polymeric chains as well as owing to the steric hindrance to NPs aggregation, produced by the hexamethylene fragments. The ionene-modified gold NPs are stable in solution at least for 4 month. Their destabilization can be achieved in the presence of substances decreasing the surface potential of NPs or able to interact with two or more NPs, leading to their cross-linking. In this concept, some negatively charged species are of interest as they can effectively interact with positively charged NPs.
When adding different anions up to the concentration of 0.25 mg mL-1, it was found that only sulfate caused aggregation of NPs. Dihydrophosphate, nitrate, ethylenediaminetetraacetate, chlorate, perchlorate, bromide, fluoride, chloride and hydrocarbonate did not affect the NPs aggregative state remarkably. The aggregation became apparent with the change of color of the solution from ruby to blue, and could be characterized by the absorbance ratio at 650 and 520 nm – A650/A520 (Fig. 2).
The electron spectrum of NPs solution had a new band at 700 nm, which corresponded to NPs aggregates, the SPR band of individual NPs at 520 nm was decreased. TEM-image of the sample showed the presence of differently shaped bunch-like aggregates, whereas ED pattern had no change in the stimuli positions that proved immutability of a gold core during the interaction (Fig. 3).
We assume that the effect observed is connected with the different charge and size of these anions, and hence, the different ability to form bonds with positively charged AuNPs.
The sorption of sulfate on the NP surface results in decreasing positive charge and repulsion of NPs as well as gives rise to their crosslinking via negatively charged sulfate and positively charged ionene. Both these factors promote aggregation.
3.1. Influence of different factors on aggregation of NPs in the presence of sulfate
The influence of the time of interaction, pH and the concentrations of NPs and sulfate on the aggregation was investigated. The degree of aggregation was estimated by comparing the absorption spectra and ratio of absorbances at wavelengths corresponding to the aggregates and individual NPs A650/A520. According to the literature [10, 40], this ratio is often used for calibration curve plotting when determining metals and organic compounds.
Influence of the time of interaction. The duration of interaction between NPs and sulfate strongly effects on the aggregative ratio A650/A520. The dependence of this ratio on the time of interaction is given in Fig. 4. One can see that the interaction proceeds in less than 10 min. This time interval allows getting maximum yield of AuNPs aggregates. However, taking into account the task to develop a simple and quick analytical procedure for the determination of sulfate, in all further experiments the time of interaction was chosen as 2 min. It is quite enough to achieve the reasonable signal and to accelerate the procedure by more than 4 times.
Influence of pH. The dependence of the NPs aggregation on pH in absence and in the presence of sulfate was investigated. The acidity required was adjusted by H3PO4 and NaOH solutions. Corresponding dependences are depicted in Fig. 5. According to the figure, sulfate causes aggregation at pH 3.5–11. The decrease in the effect of sulfate ions at pH lower than 3.5 seems to be connected with their protonation (pKa ≈ 2 for HSO4-) leading to decrease in the charge. At pH > 11, one can see the dramatic increase in the aggregation rate even in absence of sulfate, which is probably connected with destabilization of NPs by OH- ions. The wide interval of the stable effect of sulfate is favorable for the analysis of most real samples. In all further investigations pH was not adjusted.
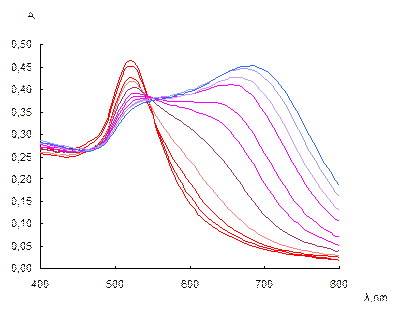
Influence of the sulfate concentration. The absorption spectra of NPs solutions at different sulfate concentrations are given in Fig. 6a. It can be seen that when the concentration of sulfate is increased, a monotonous decrease in the surface plasmon resonance band of individual NPs at 520 nm and an increase in the NPs aggregates band are observed. The wavelength of the aggregates band is gradually shifted from 620 to 690 nm, as a result of the aggregates enlargement. Fig. 6b demonstrates the dependence of the A650/A520 ratio on the concentration of sulfate. Sulfate causes aggregation of NPs at the concentrations higher than 0.06 mg mL-1. The ratio A650/A520 depends near linearly on the sulfate concentration in a range 0.07–0.13 mg mL-1. (R2 = 0.979). At the concentrations higher than 0.15 mg mL-1, maximum aggregation is observed.
Influence of the NPs concentration. The dependence of the A650/A520 ratio on the concentration of sulfate at the different NPs concentrations (0.06, 0.12 and 0.25 mmol L-1 Au) is shown in Fig. 7. It can be seen that the concentration of NPs practically does not affect the aggregation. The low as well as the high concentration of NPs can produce an increase in the errors connected with measurement of the absorbance. Therefore, we chose the concentration of 0.12 mmol L-1 Au as the optimal one (absorbance at 520 nm ≈ 0.45).
3.2. Analytical features of the method
The dependence of the A650/A520 ratio on the concentration of sulfate enables to develop an analytical procedure for its spectrophotometric determination using 6,6-ionene stabilized gold NPs. The analytical features of the method are as follows: limit of detection 0.06 mg mL-1, analytical range 0.07 – 0.13 mg mL-1, relative standard deviation 5 % (calculated for 0.1 mg mL-1 of sulfate). The detection limit was estimated as an abscissa of the intercept point of tangents to the linear parts of the dependence of the A650/A520 ratio on the concentration of sulfate (see Fig. 6b).
The selectivity of the method was evaluated. It was found that the determination of 0.1 mg mL-1 of sulfate was not affected by at least equal amounts of Cl-, HCO3-, H2PO4-, Na++K+, and 0.03 mg mL-1 of Ca2+ and Mg2+.
It should be mentioned that an important feature of the developed method is easiness in carrying out a semiquantitative test-determination. The colors of the NPs in absence and in the presence of sulfate are in contrast and can be easily distinguished by naked eye.
To show clearly the advantages of proposed method in respect of the analysis of samples with the relatively high amounts of sulfate, we considered it in comparison with a method similar to [38], when applied to the analysis of a water sample containing 2.0 mg mL-1 sulfate. Some features of both methods in this concept are represented in Table 1. It can be seen that the proposed method has 3 times less number of operations prior to the analytical procedure (single-step determination), requires 15 times less time, no additional deionized water and 10 times less consumption of gold at the comparable reproducibility. Moreover, visual effects at the concentration of sulfate in a sample below (1.5 mg mL-1) and above (2.5 mg mL-1) normalized value (which was chosen as 2.0 mg mL-1) are more distinguished for the proposed method (crimson and blue) than for the method of comparison (crimson and violet). Besides, the proposed method does not require additional buffer and decreased temperatures of the NPs storage.
In order to test the feasibility of the proposed method in real samples, we used ionene-stabilized AuNPs probe for the direct measuring of SO42- in mineral waters. The unknown concentrations of SO42- in the samples were found by ion chromatography. The results are listed in Table 2.
4. Conclusions
It has been demonstrated that 6,6-ionene is a promising cationic polymer for synthesis of stable positively charged gold nanoparticles that seems to be connected with their both electrostatic and steric stabilization by this substance. These desensitized nanoparticles shown to have selectivity to sulfate and can be used for the rapid single-step detection of the quite high concentrations of sulfate in real samples without any pretreatment and dilution of a sample. The procedure is applicable in the wide pH range of a sample. It can be easily realized in the variant of a semiquantitative test.
Acknowledgments
The work was financially supported by the Russian Foundation for Basic Research (grants N 13-03-00100, 14-03-31109)
We also thank MSU Joint Use Center and Dr. Sergey S. Abramchuk for recording TEM images and ED patterns of the samples, Dr. Elena N. Shapovalova and Anna N. Ioutsi for providing us with the 6,6-ionene samples, Dr. Alexandra F. Prokhorova for carrying out the ion chromatography determination.
References
[1] M. Cuartero, M.S. Garcia, J.A. Ortuno, Electrochim. Acta, 93 (2013) 272.
[2] M.R. Ganjali, L. Naji, T. Poursaberi, M. Taghizadeh, H. Pirelahi, M. Yousefi, A. Yeganeh-Faal, M. Shamsipur, Talanta, 58 (2002) 359.
[3] V.V. Egorov, V.A. Nazarov, E.B. Okaev, T.E. Pavlova, J. Anal. Chem., 61 (2006) 382.
[4] J.A. Morales, L.S. Graterol, J. Mesa, J. Chromatogr. A, 884 (2000) 185.
[5] J.W. O’Reilly, G.W. Dicinoski, M.J. Shaw, P.R. Haddad, Anal. Chim. Acta, 432 (2001) 165.
[6] M. Zhou, D. Guo, Microchem. J., 65 (2000) 221.
[7] Y. Miura, Y. Matsushita, P.R. Haddad, J. Chromatogr. A, 1085 (2005) 47.
[8] S.-I. Ohira, K. Toda, J. Chromatogr. A, 1121 (2006) 280.
[9] S.C. Stefanovich, T. Bolanca, L. Churkovich, J. Chromatogr. A, 918 (2001) 325.
[10] L. Guo, J. Zhong, J. Wu, F. Fu, G. Chen, X. Zheng, S. Lin, Talanta, 28 (2010) 1654.
[11] V.V. Apyari, S.G. Dmitrienko, Arkhipova V.V., Atnagulov A.G., Zolotov Yu.A., Anal. Methods, 4 (2012) 3193.
[12] P. Rezanka, H. Rezankova, P. Matějka, V. Kral, Colloid. Surface A, 364 (2010) 94.
[13] J. Zhang, L.H. Wang, D. Pan, S.P. Song, F.Y.C. Boey, H. Zhang, C.H. Fan, Small 4 (2008) 1196.
[14] Y.F. Zhang, B.X. Li, X.L. Chen, Microchim. Acta, 168 (2010) 107.
[15] J. Wang, L.H. Wang, X.F. Liu, Z.Q. Liang, S.P. Song, W.X. Li, G.X. Li, C.H. Fan, Adv. Mater. 19 (2007) 3943.
[16] Y. Jiang, H. Zhao, Y.Q. Lin, N.N. Zhu, Y.R. Ma, L.Q. Mao, Angew. Chem.-Ger. Edit., 49 (2010) 4800.
[17] T. Li, K. Zhu, S. He, X. Xia, S. Liu, Z. Wang, X. Jiang, Analyst, 136 (2011) 2893.
[18] Y. Zhang, B. Li, C. Xu, Analyst, 135 (2010) 1579.
[19] J.M. Slocik, J.S.Jr. Zabinski, D.M. Phillips, R.R. Naik, Small, 4 (2008) 548.
[20] N. Dinga, H. Zhaoa, W. Penga, Y. Hea, Y. Zhoua, L. Yuana, Y. Zhang, Colloid. Surface A, 395 (2012) 161.
[21] Z. Liu, J. Hua, S. Tong, Q. Cao, H. Yuan, Spectrochim. Acta A, 97 (2012) 737.
[22] N. Bi, Y. Chen, H. Qi, X. Zheng, Y. Chen, X. Liao, H. Zhang, Y. Tian, Spectrochim. Acta A, 95 (2012) 276.
[23] N. Bi, Y. Chen, H. Qi, X. Zheng, Y. Chen, X. Liao, H. Zhang, Y. Tian, Sensor. Actuat. B-Chem., 166-167 (2012) 766.
[24] L. Zhao, Y. Jin, Z. Yan, Y. Liu, H. Zhu, Anal. Chim. Acta 731 (2012) 75.
[25]. J. Xin, L. Miaî, S. Chen, A. Wu, Anal. Method. Instrum., 4 (2012) 1259.
[26] C. Hua, W.H. Zhang, S.R.M. Almeida, S. Ciampi, D. Gloria, G. Liu, J.B. Harper, J.J. Gooding, Analyst, 137 (2012) 82.
[27] Q. Shen, W. Li, S. Tang, Y. Hu, Z. Nie, Y. Huang, S. Yao, Biosens. Bioelectron., 41 (2012) 663.
[28] R. Velu, V.T. Ramakrishnan, P. Ramamurthy, Tetrahedron Lett., 51 (2010) 4331.
[29] S. Kado, A. Furui, Y. Akiyama, Y. Nakahara, K. Kimura, Anal. Sci. 25 (2009) 261.
[30] W. Liu, Z. Dub, Y. Qian, F. Li, Sensor. Actuat. B-Chem., 176 (2013) 927.
[31] Y. Kubo, S. Uchida, Y. Kemmochi, T. Okubo, Tetrahedron Lett., 46 (2005) 4369.
[32] S. Watanabe, H. Seguchi, K. Yoshida, K. Kifune, T. Tadaki, H. Shiozaki, Tetrahedron Lett., 46 (2005) 8827.
[33] T. Minami, K. Kaneko, T. Nagasaki, Y. Kubo, Tetrahedron Lett., 49 (2008) 432.
[34] M.H. Kim, S. Kim, H.H. Jang, S. Yi, S.H. Seo, M.S. Han, Tetrahedron Lett., 51 (2010) 4712.
[35] W.L. Daniel, H.S. Han, J.S. Lee, C.A. Mirkin, J. Am. Chem. Soc., 131 (2009) 6362.
[36] A. Pandya, K.V. Joshi, N.R. Modi, S.K. Menon, Sensor. Actuat. B, 168 (2012) 54.
[37] J. Zhang, X. Wang, X. Yang, Analyst, 137 (2012) 2806.
[38] M. Zhang, Y.-Q. Liu, B.-C. Ye, Analyst, 136 (2011) 4558.
[39] A.V. Pirogov, M.M. Platonov, O.A. Shpigun, J. Chromatogr. A, 850 (1999) 53–63.
[40] F. Tan, X. Liu, X. Quan, J. Chen, X. Li and H. Zhao, Anal. Methods, 3 (2011) 343–347.
a b


c
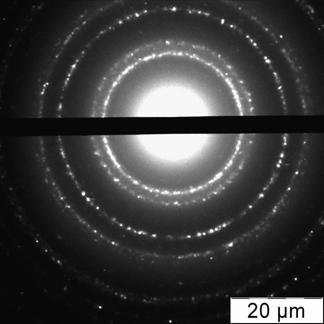
Fig. 1. Absorption spectrum (a), TEM image (b) and electron diffraction pattern (c) of the ionene-stabilized gold NPs
a: cNPs = 0.12 mmol L-1 Au
![]()
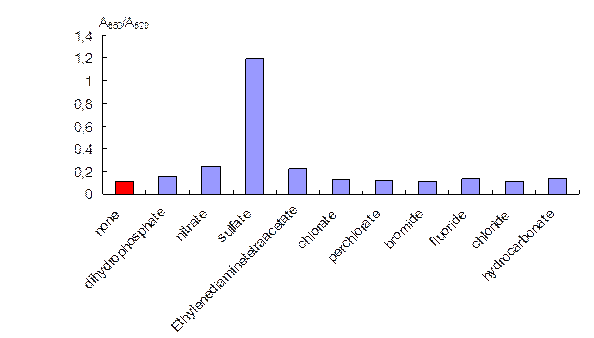
Fig. 2. The influence of different anions on the aggregation ratio of ionene-stabilized NPs.
canion = 0.25 mg mL-1, cNPs = 0.12 mmol L-1 Au.
a b
![]()
![]()
![]() 2
2
![]() 1
1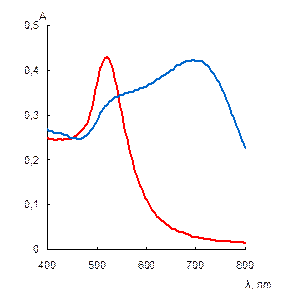
c
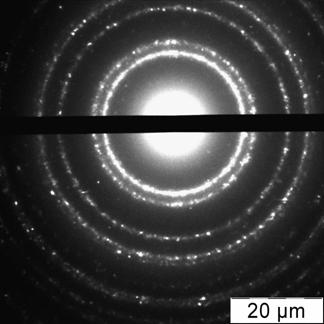
Fig. 3. Absorption spectrum and color of the ionene-modified gold NPs water solution in absence (1) and in the presence (2) of 0.25 mg mL-1 sulfate (a), TEM image (b) and ED pattern (c) of the NPs after interaction with sulfate. cNPs = 0.12 mmol L-1 Au
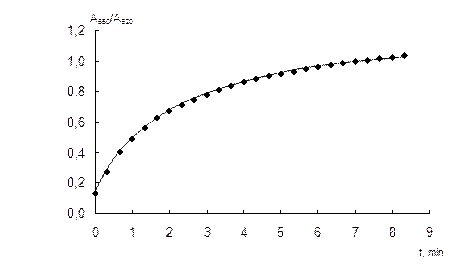
Fig. 4. Dependence of the aggregative ratio (A650/A520) of the nanoparticles in the presence of 0.1 mg mL-1 sulfate on the time of interaction.
cNPs = 0.12 mmol L-1 Au
2 1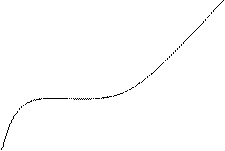
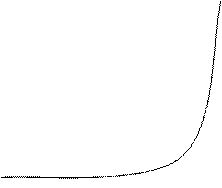
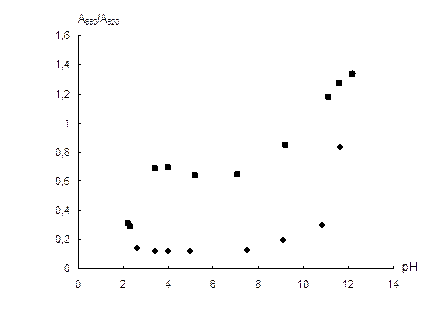
Fig. 5. Dependence of A650/A520 ratio on pH in absence (1) and in the presence (2) of 0.1 mg mL-1 sulfate.
cNPs = 0.12 mmol L-1 Au, t = 2 min.
a
Ñsulfate
![]()
b
LOD![]()
![]()

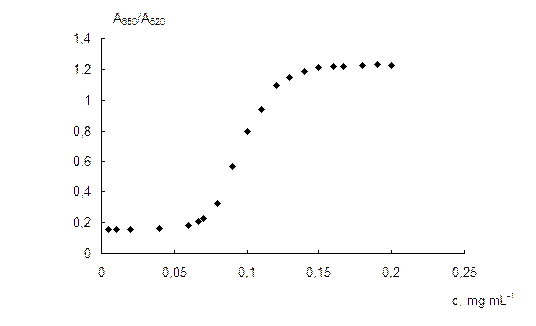
Fig. 6. Absorption spectra of NPs solutions at different sulfate concentrations (a) and dependence of the A650/A520 ratio on the concentration of sulfate (b). cNPs = 0.12 mmol L-1 Au, t = 2 min; a: csulfate, mg mL-1: 0, 0.06, 0.07, 0.08, 0.09, 0.1, 0.11, 0.12, 0.13, 0.14, 0.15.
1 3 2
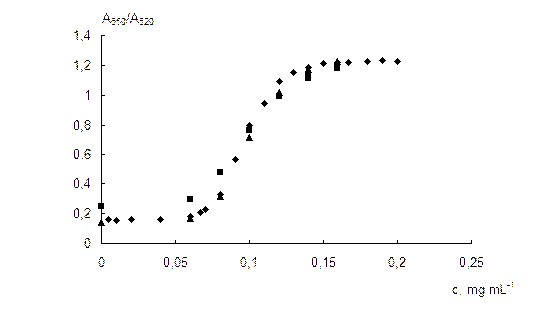
Fig. 7. The dependence of the A650/A520 ratio on the concentration of sulfate at the different concentrations of NPs. cNPs, mmol L-1 Au: 0.06 (1), 0.12 (2) and 0.25 (3).
Table 1. Some features of the proposed method compared with those of a method similar to [38] in respect of the analysis of a water sample containing 2.0 mg mL-1 sulfate.
|
Feature |
Method similar to [38] |
Proposed method |
||||||
|
Typical procedure of the analysis of a water sample containing 2.0 mg mL-1 sulfate |
Sequence of the operations |
Typical time of the operation, min |
Typical RSD of the operation, % |
Sequence of the operations |
Typical time of the operation, min |
Typical RSD of the operation, % |
||
|
1) Taking of the 1 mL aliquot, transferring into the 100 mL volumetric flask |
0.1 |
0.6 |
1) Taking of the 0.15 mL aliquot, transferring into the cuvette |
0.1 |
1.0 |
|||
|
2) Dilution with deionized water up to the mark, homogenization |
1 |
0.1 |
2) The analytical procedure |
2 |
5 |
|||
|
3) Taking of the 0.01 mL aliquot, transferring into the microtiter plate |
0.1 |
1.2 |
|
|
|
|||
|
4) The analytical procedure |
30 |
3.6 |
|
|
|
|||
|
Number of operations prior to the analytical procedure |
3 |
1 |
||||||
|
Total time of the analysis, min |
31.2 |
2.1 |
||||||
|
|
3.8 |
5.1 |
||||||
|
Expanses in additional deionized water, mL |
99 |
0 |
||||||
|
Expanses in AuNPs (for spectrophotomet-ric measurements in a 1 cm cuvette with 3 mL of a solution), mg |
0.74 |
0.07 |
||||||
|
Visual effect at the concentration of sulfate in a sample below (1.5 mg mL-1) and above (2.5 mg mL-1) normalized value of 2.0 mg mL-1 |
1.5 mg mL-1
|
2.5 mg mL-1
|
1.5 mg mL-1
|
2.5 mg mL-1
|
||||
|
Additional conditions |
Acetate buffer required, AuNPs are stored at 4 ˚C |
Buffer is not required, AuNPs are stored at the room temperature |
||||||
Table 2. Determination of SO42- in mineral water samples (n = 3, P = 0.95)
|
Sample |
Found in the sample with the independent method, mg mL-1 |
Volume taken for the analysis by proposed method, mL |
Added sulfate, mg mL-1 |
Found sulfate, mg mL-1 |
RSD, % |
|
Mineral water «Stelmas» |
2.2 ± 0.2 |
0.1 |
0 |
2.5 ± 0.3 |
5 |
|
0.6 |
3.2 ± 0.5 |
6 |
|||
|
Mineral water «Novoterskaya» |
1.2 ± 0.1 |
0.2 |
0 |
1.4 ± 0.2 |
5 |
|
0.25 |
1.7 ± 0.3 |
6 |