Label-free gold nanoparticles for the determination of neomycin
Lomonosov Moscow State University, Chemistry Department, Leninskie gory, 1/3, 119991 Moscow, Russia
Your Dedicated Software Development Company
Graphical abstract

Abstract
A new spectrophotometric method for the determination of neomycin has been developed. The method is based on aggregation of label-free gold nanoparticles leading to change in absorption spectra and color of the solution. Influence of different factors (the concentration of ethylenediaminetetraacetate (EDTA), pH, the concentrations of neomycin and the nanoparticles) on the aggregation and analytical performance of the method was investigated. EDTA plays an important role not only as a masking agent to eliminate interferences of metal cations but strongly affects the sensitivity of the nanoparticles relative to neomycin. The method allows to determine neomycin with detection limit of 28 ng·mL-1. It was applied to analysis of eye- and ear-drops. The sample pretreatment is simply done by diluting the formulation with water.
Keywords: gold nanoparticles, neomycin, determination, surface plasmon resonance, spectrophotometry
1. Introduction
Neomycin belongs to a chemical class of aminoglycoside antibiotics produced from actinomycete Streptomyces fradiae. It is often used in a variety of antibiotic pharmaceutical products. This antibiotic poseses good antibacterial activity with respect to gram-negative and gram-positive bacteria. However, aminoglycosides (and especially neomycin) cause some toxic effect such as oto- and nephrotoxicity. Uncontrolled using aminoglycoside antibiotics in medicine and veterinary for infective diseases treatment results in their accumulation in different objects and may be dangerous for people’s health. Therefore, it is important to develop sensitive, selective, simple and cost-effective methods for the determination of neomycin [1].
Different analytical methods for the determination of neomycin have been described such as HPLC [2 – 10], capillary electrophoresis [11, 12], electrochemical methods [13], immuno assays [14, 15]. Neomycin has no absorption ability in UV–vis range, and most determinations have to use different kinds of derivatization or indirect approach [11]. These procedures often are either complex and expansive or time-consuming. Only one work found deals with direct UV-detection of neomycin at 200 nm in capillary electrophoresis [12]. The same reason restrains a development of sensitive and simple spectrophotometric methods for the determination of neomycin. Only one spectrophotometric procedure of neomycin determination was found in the literature of the last years [16]. This method is based on ion-association of neomycin with chromotropic acid azo-derivatives.
Gold nanoparticles have been suggested recently as peculiar chromogenic reagents [17 – 19]. Their optical properties are conditioned by surface plasmon resonance (SPR) that appears as an intense absorbance band in the visible range [20]. Its position strongly depends on aggregative state of the nanoparticles. The change in color during aggregation process is contrasting and can be used for detection of an analyte. This optical phenomenon has been already used for the determination of different metal ions [21], anions [22 – 26] and organic compounds [27 – 33].
The aim of this work was to develop a simple and cost effective spectrophotometric method for the determination of neomycin using label-free gold nanoparticles.
2. Material and methods
2.1. Materials
Chemically pure hydrogen tetrachloroaurate, sodium citrate and analytical grade neomycin sulfate, erythromycin, ampicillin, oxytetracycline, sulfamethazine, ethylenediaminetetraacetate disodium salt (EDTA), sodium hydroxide and hydrochloric acid were used. The substances stock solutions were prepared by dissolving their weighted portions in deionized water; stock 0.25 mol L-1 solution of EDTA was prepared by dissolving the weighted portion of EDTA in 0.25 mol L-1 NaOH.
2.2. Instrumentation
Absorption spectra of solutions were recorded by SF-103 spectrophotometer (Akvilon, Russia), pH was measured by Ekspert 001 ion meter (Ekoniks, Russia). TEM-images of the samples were recorded using transmission electron microscope LEO912 AB OMEGA (Carl Zeiss, Germany). Chromatographic determination of neomycin in eye- and ear-drops was performed using the PerkinElmer Series 200 system with a refractometric detector on Thermo Scientific Hypersil Gold column (50×4.6); the sample volume was 5 μL; eluent – water solution containing 260 mmol L-1 trifluoroacetic acid and 75 mmol L-1 sodium hydroxide; flow rate was equal to 0.7 mL min-1.
2.3. Synthesis of label-free nanoparticles
Label-free nanoparticles (NPs) were prepared by reducing metal salt precursor (hydrogen tetrachloroaurate, HAuCl4) in a liquid phase by citrate according to the Frens method [34] with a slight modification.
Briefly, 1 mL of 1 % HAuCl4 was introduced in 250 mL bulb, diluted with 100 mL of deionized water and heated until boiling. 1.4 mL of 1 % sodium citrate was added to the hot solution at stirring. The solution was boiled in 5 min till stable ruby color. The mixture was cooled at stirring and kept in the dark for 24 hours to complete stabilization and re-crystallization of NPs. The concentration of NPs in the final solution was 70 mg·mL-1 (0.35 mM in terms of gold).
It has been shown in the previous work [35] that gold NPs obtained as described above have surface plasmon resonance band at 520 – 530 nm in the absorption spectra of their water solutions. Nanoparticles of 18 – 26 nm predominate in the solution, the average diameter of NPs being 23 nm.
2.4. Determination of neomycin
To construct a calibration curve, 0 – 25 ng of neomycin were introduced in a test-tube and diluted with deionized water up to 3.9 mL. Then 0.1 mL of 0.25 mol L-1 EDTA and 1 mL of NPs solutions were added successively. The final volume was 5 mL. Absorption spectra were recorded after 3 min of the reaction. The calibration curve was constructed as À700/À520 ratio versus the concentration of neomycin.
3. Results and discussion
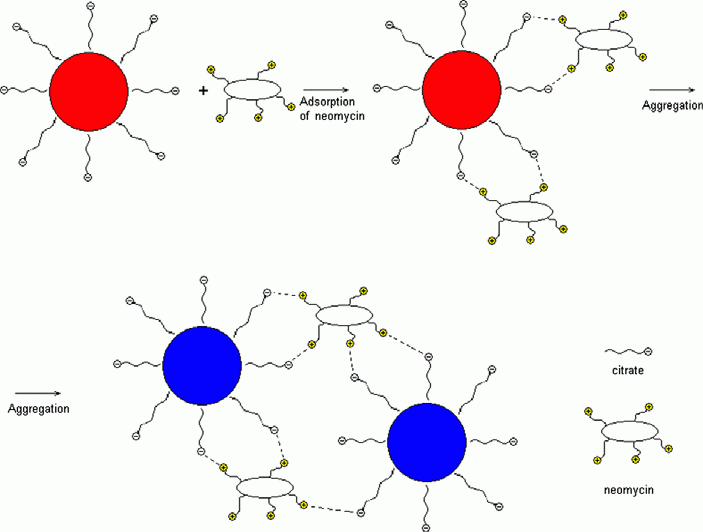
The label-free gold NPs are negatively charged in the solution due to the dissociation of citrate on their surface. This charge results in electrostatic repulsion of the particles that prevents their aggregation. Therefore, some positively charged species decreasing the charge of NPs would decrease repulsion of the NPs and can cause aggregation. We investigated the influence of antibacterials representatives of different classes on the NPs. There were the representatives of aminoglycosides (neomycin), macrolides (erythromycin), penicillins (ampicillin), tetracyclines (oxytetracycline) and sulfanilamides (sulfamethazine). Addition of different antibiotics showed that only neomycin caused aggregation of NPs, being already at the concentration of 40 ng·mL-1 (Fig. 1). Erythromycin, ampicillin, oxytetracycline and sulfamethazine did not remarkably affect NPs aggregative state even at concentration higher 140 ng·mL-1. Aggregation resulted in appearing the absorbance band of NPs aggregates at 700 nm and decreasing the band of single NPs at 520 nm (Fig. 1). The color of the solution was changed from ruby to blue. TEM-images of the solution after addition of neomycin showed the presence of NPs aggregates of different shape and size. Several aggregates and their parts had prominent chain shape (Fig. S1).
This effect can be ascribed to different charges of the substances owing to their different ionic state under the experimental conditions. A neomycin molecule contains 6 aminogroups. Calculation of pKa meanings for neomycin made using ACD Labs® software yields ðÊà=6.6; 8.0; 8.0; 9.3; 9.5 and 9.5 for its protonated form. It means that at least 5 of 6 aminogroups are protonated under the experimental conditions (pH 7.5). Therefore the molecule carries high positive charge distributed in its different parts. In this context, neomycin has a high aggregative potential with respect to the NPs. Moreover, the distribution of charge makes it similar to peculiar “glue” possible to bind the nanoparticles together.
Adsorption of neomycin on the NP surface results in essential decrease in the negative charge of NPs and their aggregative stability. Another factor that probably increases aggregation is a possibility of NPs interparticle crosslinking owing to interaction of positively charged ammonium groups of neomycin with citrate anions on surfaces of the two different NPs. Aggregation of NPs under the influence of neomycin can be described by Scheme S1.
It is well known that aggregation of NPs can also be induced by metal cations, especially multicharged. To eliminate the interferences caused by these ions by catching them in negatively charged complexes, we added EDTA into the solution as a masking agent [35]. The same approach was used by us in this work.
3.1. Influence of different factors on aggregation of NPs under the influence of neomycin
It has been investigated the influence of EDTA concentration, ionic strength, pH, concentrations of NPs and neomycin on the aggregation. To estimate the degree of aggregation we compared the absorption spectra and ratios of absorbances at wavelengths corresponding to the aggregates and individual NPs À700/À520 [22, 32, 35].
Influence of EDTA. In the present study we investigated the influence of EDTA on the interaction of gold NPs with neomycin. It was found that EDTA essentially affects the aggregation of NPs under the influence of neomycin. Fig. 2 shows dependences of À700/À520 ratio on the concentration of neomycin at the different concentrations of EDTA. It can be seen that the bigger concentration of EDTA in the solution, the lower amounts of neomycin cause aggregation of the NPs. This effect may stems from the decrease of the colloid system stability because of the increasing of ionic strength when adding EDTA. Such effects of electrolytes on NPs have been described in the literature [36]. They are remarkable at the ionic strength 10 – 40 mmol L-1 depending on a type of the nanoparticles. This supposition is indirectly proved by the fact that at the EDTA concentrations higher 10 mmol L-1 aggregation was observed even in the absence of neomycin.
It was studied the influence of ionic strength made by NaCl on the NPs in the solution (Fig. S2). One can see that ionic strength higher than 30 mmol L-1 caused aggregation of the NPs. Comparing with the EDTA which at pH 7.5 exists as Na3Y salt, we can notice that the aggregation appears at the same concentrations of Na+ ions (30 mmol L-1).
We suppose that sodium cations play the key role in these processes penetrating the layer of counter-ions of a negatively charged nanoparticle and decreasing its charge and ζ-potential. This leads to the decrease in electrostatic repulsion of NPs which increases “sensitivity” of the system to the presence of substances able to cause aggregation. The concentration of neomycin required for achieving half of the maximal response (cneom50%) depends on the concentration of EDTA near linearly (Fig S3). It can be used to choose the concentration of EDTA necessary to determine neomycin with the required sensitivity. In all further experiments the concentration of EDTA was chosen as 5 mmol L-1
Influence of pH. The dependence of NPs aggregation in the presence of neomycin on pH was investigated. The acidity required was adjusted by HCl and NaOH solutions. Fig. 3 represents the dependence of A700/A520 ratio on pH in the absence and in the presence of neomycin as well as corresponding difference between the signals. One can see that neomycin causes aggregation at pH 4 – 8. In the acidic medium hydrogen ions lead to aggregation even without neomycin. At pH > 8 aggregation is suppressed that may be a consequence of partial deprotonation of neomycin and of the increase in dissociation degree of citrate ions on the NPs surface leading to increase in their charge and stability of the system. However when choosing the optimal pH value one should keep in mind of necessity to retain of maximal amount of interfering ions in the complexes with EDTA. It can be achieved at high pH. That is why in all further experiments pH was adjusted to 7 – 8.
Influence of the neomycin concentration. As it has been stated, the concentration neomycin affect essentially aggregative state of the gold NPs. Absorption spectra of NPs solutions at the different concentration of neomycin are given in Fig. 4a. One can see that the bigger concentration of neomycin is, the lower the surface plasmon resonance band of individual NPs at 520 nm. At the same time an increase in NPs aggregates band at 700 nm is observed. The dependence of À700/À520 ratio on the concentration of neomycin represents a sharp S-shaped curve with a linear part in a range 30 – 40 ng mL-1 of neomycin.
3.2. Analytical features of the method
The linear part of the dependence of A700/A520 ratio on the concentration of neomycin in a solution can be used for its spectrophotometric determination. The analytical features of the determination are given in Table 1. Detection limits were calculated as an abscissa of intercept point of tangents to the linear parts of dependence of À700/À520 ratio on the concentration of neomycin (see Fig. 4b, shown as dotted lines).
The analytical range is narrow at the low concentration of NPs and is wider at the high concentration of NPs, however the limit of detection is bigger. The use of the NPs concentrations less than 0.07 mmol L-1 is not preferable because of low absorbances and high errors of their measurement. Reproducibility of the determination was found to be not more than 5 %.
It was found that the determination of 35 ng·mL-1 of neomycin was no affected by at least 400-fold amount of HCO3-, 200-fold amount of SO42-, 100-fold amount of Ca2+, Mn2+, Fe3+, Cu2+, Zn2+, Cl- and 50-fold amount of Na+, K+, Mg2+.
3.3. Application to real samples
The method developed was applied to the determination of neomycin in eye- and ear-drops. The samples preparation included only successive dilution with deionized water. An aliquot of the diluted sample was transferred into test-tube and processed as described in the Experimental section. The results are given in Table 2. They are in accordance with the data declared by the producers and the results of independent HPLC determination with refractometric detection.
A comparison of the suggested method with other methods for the determination of neomycin is represented in Table 3.
4. Conclusions
The effect of aggregation of label-free gold NPs in the presence of neomycin has been studied. It leads to the change in their optical properties and color of the solution and can be used for the determination of neomycin by spectrophotometry. It has been shown that EDTA can be used not only as a masking agent to eliminate interferences of metal cations but allows to change sensitivity of the determination. When the concentration of EDTA is increased from 0 to 10 mmol L-1, the detection limit is decreased from 50 to 1 ng mL-1. The sensitivity can be increased by decreasing the concentration of the NPs. The optimal conditions of the determination have been achieved in 5 mM EDTA at pH 7 – 8 and the concentration of NPs 0.07 mmol L-1.
Under the optimal conditions the detection limit was 28 ng·mL-1.
The method has been applied to the analysis of eye- and ear-drops. The main advantages of the procedure are sensitivity, quickness, cost-effectiveness and applicability to semi-quantitative test-determinations.
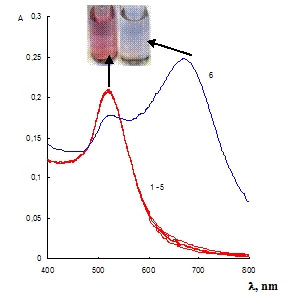
Fig. 1 Absorption spectra of label-free gold NPs water solutions in the absence of antibiotics (1) and in the presence of erythromycin (400 ng mL-1), ampicillin (190 ng mL-1), oxytetracycline (250 ng mL-1), sulfamethazine (140 ng mL-1) (2 – 5) and neomycin (40 ng mL-1). cNPs = 0.07 mmol L-1 Au, cEDTA = 5 mmol L-1, pH 7.5
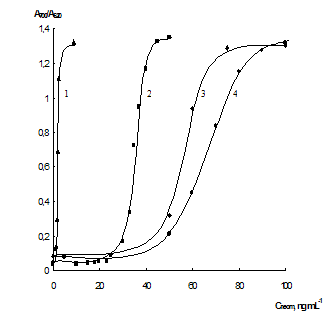
Fig. 2 Dependences of À700/À520 ratio on the concentration of neomycin at the different concentration of EDTA. CEDTA, mmol L-1: 10 (1), 5 (2), 1 (3) and 0 (4); pH 7.5
a) b)
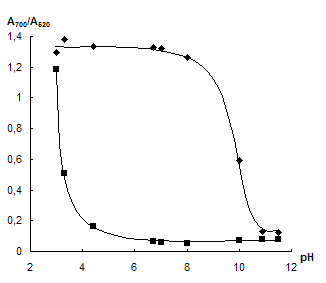
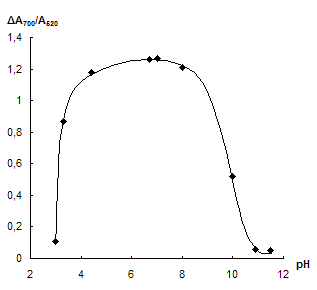
Fig. 3 Dependence of À700/À520 ratio on pH in the absence (1) and in the presence (2) of 40 ng·mL-1 neomycin (a) and corresponding difference of the signals (b). cNPs = 0.07 mmol L-1 Au, cEDTA = 5 mmol L-1
a) b)
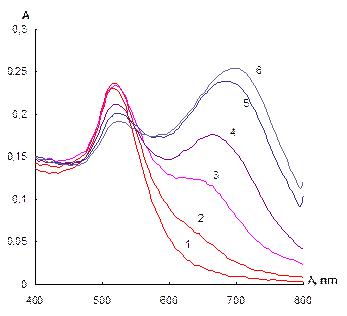
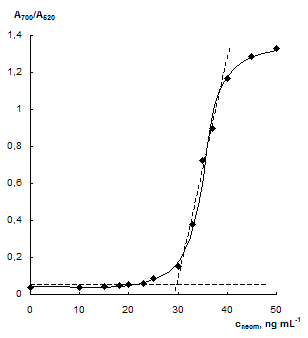
Fig. 4 Absorption spectra of NPs solutions at different neomycin concentration (a) and dependence of À700/À520 ratio on the concentration of neomycin (b). cNPs = 0.07 mmol L-1 Au, cEDTA = 5 mmol L-1, pH 7.5; a) cneom ng·mL-1: 0 (1), 30 (2), 33 (3), 35 (4), 40 (5), 45 (6)
Acknowledgements
This work was financially supported by the Russian Foundation for Basic Research (grant N 13-03-00100)
References
[1] T.A. McGlinchey, P.A. Rafter, F. Regan, G.P. McMahon, Anal. Chim. Acta 624 (2008) 1 – 15.
[2] V.P. Hanko, J.S. Rohrer, J. Pharmaceut. Biomed. Anal. 51 (2010) 96 – 102.
[3] R. Oertel, V. Neumeister, W. Kirch, J. Chromatogr. A 1058 (2004) 197 – 201.
[4] N.C. Megoulas, M.A. Koupparis, J. Chromatogr. A 1057 (2004) 125 – 131.
[5] I. Clarot, A. Regazzeti, N. Auzeil, F. Laadani, M. Citton, P. Netter, A. Nicolas, J. Chromatogr. A 1087 (2005) 236 – 244.
[6] R. Oertel, U. Renner, W. Kirch, J. Pharmaceut. Biomed. Anal. 35 (2004) 633 – 638.
[7] V.P. Hanko, J.S. Rohrer, J. Pharmaceut. Biomed. Anal. 43 (2007) 131 – 141.
[8] D.G. Mascher, C.P. Unger, H.J. Mascher, J. Pharmaceut. Biomed. Anal. 43 (2007) 691 – 700.
[9] N.H. Zawilla, J. Diana, J. Hoogmartens, E. Adams, J. Chromatogr. B 833 (2006) 191 – 198.
[10] M. Pendela, E. Adams, J. Hoogmartens, J. Pharmaceut. Biomed. Anal. 36 (2004) 751 – 757.
[11] P. Srisom, B. Liawruangrath, S. Liawruangrath, J.M. Slater, S. Wangkarn, J. Pharmaceut. Biomed. Anal. 43 (2007) 1013 – 1018.
[12] A.L. Huidobro, A. Garcia, C. Barbas, J. Pharmaceut. Biomed. Anal. 49 (2009) 1303 – 1307.
[13] Y. Ding, H. Yu, S. Mou, J. Chromatogr. A 1039 (2004) 39 – 43.
[14] H. Haasnoot, P. Stouten, G. Cazemier, A. Lommen, J.F.M. Nouws, H.J. Keukens, Analyst 124 (1999) 301 – 305.
[15] Y. Zhu, J.I. Son, Y.-B. Shim., Biosens. Bioelectron. 26 (2010) 1002 – 1008.
[16] A.S. Amin, Y.M. Issa, Spectrochim. Acta A 59 (2003) 663 – 670.
[17] X. Liu, M. Atwater, J. Wang, Q. Huo, Colloids Surf. B. 58 (2007) 3 – 7.
[18] D. Liu, Z. Wang, X. Jiang, Nanoscale 3 (2011) 1421 – 1433.
[19] Y. Sun, Y. Xia, Analyst 128 (2003) 686 – 691.
[20] S.K. Ghosh, T. Pal, Chem. Rev. 107 (2007) 4797 – 4862.
[21] Y.-W. Lin, C.-C. Huang, H.-T. Chang, Analyst 136 (2011) 863 – 871.
[22] F. Tan, X. Liu, X. Quan, J. Chen, X. Li, H. Zhao, Anal. Methods 3 (2011) 343 – 347.
[23] M. Zhang, Y.-Q. Liu, B.-C. Ye, Analyst 136 (2011) 4558 – 4562.
[24] M.H. Kim, S. Kim, H.H. Jang, S. Yi, S.H. Seo, M.S. Han, Tetrahedron Lett. 51 (2010) 4712 – 4716.
[25] J. Zhang, X. Wang, X. Yang, Analyst 137 (2012) 2806 – 2812.
[26] W.L. Daniel, M.S. Han, J.-S. Lee, C.A. Mirkin, J. Am. Chem. Soc. 131 (2009) 6362 – 6363.
[27] C.-L. Kuong, W.-Y. Chen, Y.-C. Chen, Anal. Bioanal. Chem. 387 (2007) 2091 – 2099.
[28] Y. Wang, L. Zhan, C.Z. Huang, Anal. Methods 2 (2010) 1982 – 1988.
[29] D. Liu, W. Chen, J. Wei, X. Li, Z. Wang, X. Jiang, Anal. Chem. 84 (2012) 4185 − 4191.
[30] L. Li, B. Li, Analyst 134 (2009) 1361 – 1365.
[31] Y. Li, Y. Duan, J. Li, J. Zheng, H. Yu, R. Yang, Anal. Chem. 84 (2012) 4732 − 4738.
[32] L. Guo, J. Zhong, J. Wu, F. Fu, G. Chen, X. Zheng, S. Lin, Talanta 82 (2010) 1654 – 1658.
[33] X. Liang, H. Wei, Z. Cui, J. Deng, Z. Zhang, X. You, X.-E. Zhang, Analyst 136 (2011) 179 – 183.
[34] G. Frens, Nat. Phys. Sci. 241 (1973) 20 – 22.
[35] V.V. Apyari, S.G. Dmitrienko, V.V. Arkhipova, A.G. Atnagulov, Yu.A. Zolotov, Anal. Methods 4 (2012) 3193 – 3199.
[36] P. Rezanka, H. Rezankova, P. Matejka, V. Kral, Colloid. Surface. A: Physicochem. Eng. Aspects. 364 (2010) 94 – 98.
[37] Z. Liu, S. Liu, L. Wang, J. Peng, Y. He, Spectrochim. Acta A 74 (2009) 36 – 41.